Canagliflozin Amputation Risk Calculator
This calculator helps you understand your risk factors for lower-limb amputation when taking canagliflozin (INVOKANA®). Based on evidence from major clinical studies, certain patients have a higher risk.
Answer a few questions about your health. The results will show your risk level and recommend next steps based on current medical guidelines.
Your Risk Factors
Your Risk Assessment
Next steps: Based on your risk assessment, your healthcare provider should discuss alternative treatment options.
When you're managing type 2 diabetes, choosing the right medication isn't just about lowering blood sugar. It's about balancing benefits with real, sometimes serious, risks. One drug that’s stirred up serious concern is canagliflozin - sold under the brand name INVOKANA®. Since 2017, doctors and patients have been asking: does this drug increase the chance of losing a toe, foot, or even a leg? The answer isn’t simple, but the evidence is clear enough to change how we use it.
What Happened? The CANVAS Study That Changed Everything
In 2017, the results of the CANVAS Program shook the diabetes world. This large study tracked over 10,000 people with type 2 diabetes who were taking either canagliflozin or a placebo. The findings were alarming: those on canagliflozin had nearly twice the risk of needing a lower-limb amputation compared to those on placebo. For the 300 mg dose, the rate jumped to 5.5 amputations per 1,000 patient-years - up from 2.8 in the placebo group. These weren’t random numbers. They came from real people. Many of these amputations were minor - toes or parts of the foot. But about 20% were major, above the ankle. The FDA responded quickly, adding a boxed warning - the strongest kind - to the drug label. For a while, many doctors stopped prescribing it altogether.Why Did This Happen? The Science Behind the Risk
The big question: is this a problem with all drugs in its class, or just canagliflozin? The answer matters because SGLT2 inhibitors are a whole group of diabetes drugs that work the same way - by making the kidneys flush out extra sugar. But here’s the key: canagliflozin is the only one linked to this risk. Studies on other drugs in the class - like empagliflozin (Jardiance) and dapagliflozin (Farxiga) - showed no similar spike in amputations. In fact, some showed no change at all, or even a slight drop. A 2023 meta-analysis of over 74,000 patients confirmed this: only canagliflozin carried a statistically significant increase in amputation risk. So why just this one? Researchers think it might be tied to how strongly it lowers blood pressure and body weight. Canagliflozin tends to drop systolic blood pressure by about 3.7 mmHg more than other SGLT2 inhibitors. That might reduce blood flow to already vulnerable feet in people with poor circulation - especially those with peripheral artery disease or nerve damage from diabetes.Who’s at Real Risk? It’s Not Everyone
This isn’t a risk for every person with diabetes. The data shows it’s concentrated in a smaller group:- People with existing peripheral artery disease (PAD) - about 1 in 5 with type 2 diabetes have this
- Those with diabetic neuropathy - up to half of patients lose sensation in their feet
- Anyone who’s had a foot ulcer before - 40% of them get another within a year
- Smokers - tobacco narrows blood vessels, making feet more vulnerable
- People with a history of prior amputation
The FDA Changed Its Mind - But the Warning Didn’t Disappear
In January 2020, the FDA removed the boxed warning. That made headlines. But don’t be fooled - the risk didn’t vanish. The agency just decided that the benefits - especially for patients with heart or kidney disease - outweighed the risk for many. The CREDENCE trial was key here. It showed canagliflozin cut the risk of kidney failure and heart-related death in people with diabetic kidney disease. That’s huge. So the FDA didn’t say, “It’s safe.” They said, “It’s safe for some - but you need to know the signs.” The current prescribing label still says: “Monitor for new pain, tenderness, sores, ulcers, or infections in the legs or feet.” That’s not a suggestion. It’s a requirement.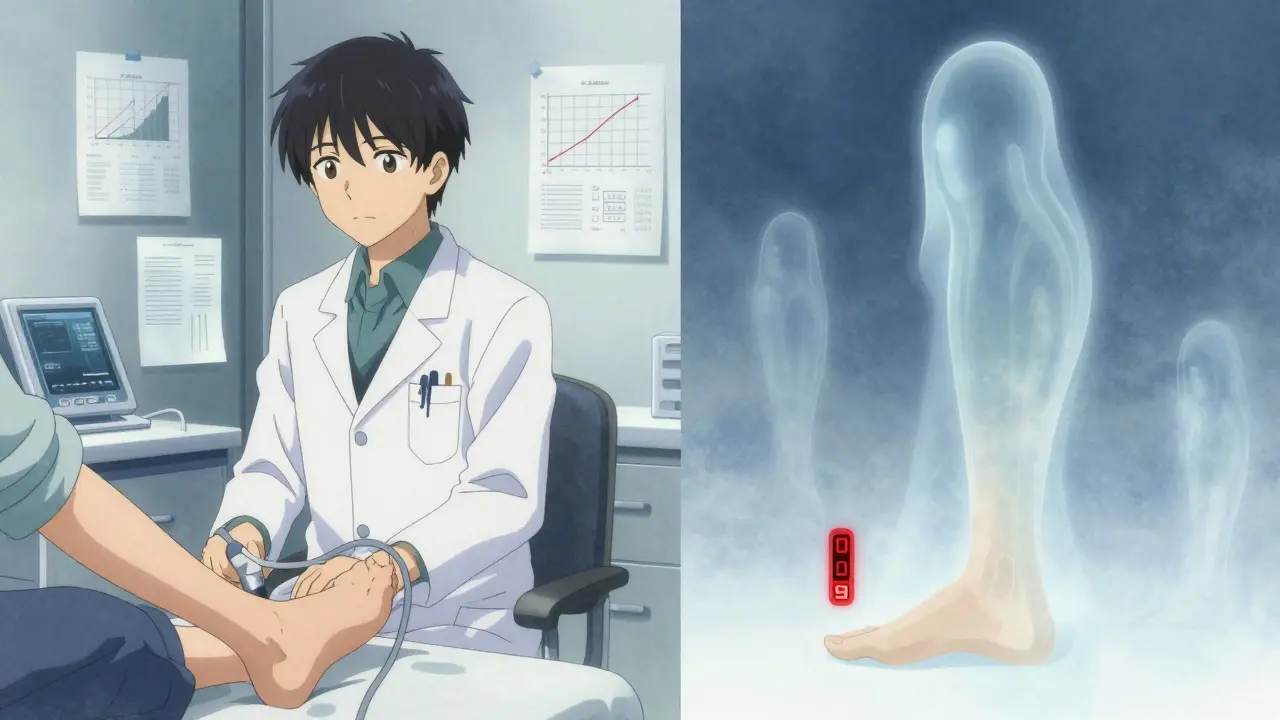
Real Stories: What Patients Are Saying
Behind every statistic is a person. On patient forums like PatientsLikeMe, about 7% of canagliflozin users reported foot problems. A small number - less than 1% - said they or someone they knew had an amputation. One Reddit user, u/DiabetesWarrior2020, shared: “After 18 months on Invokana, my podiatrist found a non-healing ulcer. I lost my toe. My endocrinologist switched me to Jardiance right away.” Another, u/SugarFreeLife, said: “I’ve been on it for three years. No foot issues. My A1c dropped from 8.5% to 6.2%.” The FDA’s own database shows a 17.8 times higher reporting rate of amputations with canagliflozin compared to empagliflozin. That’s not proof of causation, but it’s a loud signal.What Doctors Do Now: Prevention Is the Rule
Today, responsible prescribing means more than just writing a script. It means a plan:- Check your feet at every doctor visit - no exceptions
- Get an ankle-brachial index (ABI) test before starting canagliflozin if you have any heart disease or risk factors. An ABI under 0.9 means poor leg circulation - don’t start the drug
- Ask for a referral to a podiatrist if you have numbness, calluses, or sores
- Never ignore a blister, cut, or red spot on your foot - even if you can’t feel it
- Stop smoking. Period. It’s the single biggest thing you can do to protect your feet
What’s Next? New Research and Better Tools
The story isn’t over. The FOOT-STEP trial, running until 2026, is testing whether structured foot care - weekly checks, proper shoes, education - can cut amputation rates in half for people on canagliflozin. Janssen, the maker of INVOKANA, is also testing a new extended-release version. Early data suggests it might cause lower spikes in blood concentration, which could mean less impact on blood pressure and circulation. It’s still in trials, but it’s a sign the company is listening. And in 2024, the FDA required all SGLT2 inhibitors to include standardized foot care instructions in their patient medication guides. That’s progress.
Should You Still Take It?
If you’re on canagliflozin and you’re healthy - no foot problems, no smoking, no circulation issues - you’re likely fine. Many people benefit from it. It helps with weight loss, lowers blood pressure, and protects the heart and kidneys. But if you’ve had a foot sore, a numb foot, or poor circulation, you need to talk to your doctor now. Don’t wait for a wound to appear. Don’t assume it won’t happen to you. The risk is low for most, but it’s real for some - and the consequences are permanent. The best approach? Know your risk. Check your feet daily. Ask your doctor for an ABI test if you’re unsure. And if you’re starting a new diabetes drug, ask: “Is this the safest choice for my feet?”What About Other SGLT2 Inhibitors?
You don’t have to give up the benefits of this drug class. If canagliflozin isn’t right for you, here are your alternatives:- Empagliflozin (Jardiance): Proven heart and kidney protection. No increased amputation risk in large trials.
- Dapagliflozin (Farxiga): Also safe for the feet. Shown to reduce heart failure hospitalizations.
- Ertugliflozin (Steglatro): Less data, but no amputation signal so far.
Bottom Line: Awareness Saves Limbs
Canagliflozin isn’t banned. It’s not evil. It’s a tool - powerful, useful, but with a known danger zone. For the right patient, it can be life-saving. For others, it could be devastating. The difference? Awareness. Screening. Communication. Daily foot checks. No sugar-coating. If you’re on this medication, don’t panic. But don’t ignore your feet either. Your toes matter. Your mobility matters. And with the right precautions, you can keep both.Does canagliflozin cause amputations in everyone?
No. The risk is real but limited to a small subset of people - mostly those with pre-existing foot problems, poor circulation, nerve damage, or a history of ulcers. For healthy patients without these risk factors, the chance of amputation is very low.
Why was the FDA boxed warning removed if the risk still exists?
The FDA removed the boxed warning after reviewing more data, especially from the CREDENCE trial, which showed strong kidney and heart benefits in high-risk patients. They concluded that for many, the benefits outweigh the risks - but only if the patient is monitored closely. The warning was replaced with detailed precautions in the prescribing information.
Are all SGLT2 inhibitors the same when it comes to amputation risk?
No. Canagliflozin is the only SGLT2 inhibitor with consistent, statistically significant evidence linking it to higher amputation risk. Empagliflozin and dapagliflozin have not shown this signal in large trials. If foot safety is a concern, switching to one of these alternatives is a common and recommended practice.
What should I do if I notice a sore on my foot while taking canagliflozin?
Contact your doctor or podiatrist immediately - don’t wait. Even a small blister or red spot can turn into a serious infection, especially if you have nerve damage. Do not try to treat it yourself. Early intervention can prevent amputation.
Should I stop taking canagliflozin on my own if I’m worried?
No. Stopping suddenly can cause your blood sugar to spike, which is dangerous. Talk to your doctor first. They can help you switch to a safer alternative if needed, while keeping your diabetes under control.
Is there a test to check if I’m at risk before starting canagliflozin?
Yes. The ankle-brachial index (ABI) test measures blood pressure in your legs compared to your arms. An ABI below 0.9 indicates poor circulation and is considered a relative contraindication to canagliflozin. Many doctors now recommend this test before prescribing it, especially if you have heart disease or are over 50.






15 Comments
Jonathan Morris
December 17, 2025Let’s be clear: the FDA didn’t ‘remove’ the boxed warning because the risk disappeared. They removed it because the pharmaceutical industry lobbied harder than the podiatrists. Canagliflozin’s mechanism-massive osmotic diuresis-directly reduces perfusion in distal capillaries. That’s not a side effect. It’s a biomechanical inevitability for diabetics with microvascular disease. The CREDENCE trial? A cherry-picked subset. They excluded patients with ABI < 0.9. That’s not science. That’s corporate risk management dressed as clinical guidance.
And don’t tell me ‘monitor your feet.’ I’ve seen the foot clinics. They’re understaffed, underfunded, and overwhelmed. A 30-second visual check doesn’t replace Doppler studies. The real scandal? The FDA still allows this drug to be marketed as ‘heart-protective’ while the amputation risk remains unquantified in real-world populations. This isn’t pharmacology. It’s actuarial calculus disguised as medicine.
Anna Giakoumakatou
December 18, 2025Oh, so now we’re supposed to be *grateful* that Big Pharma didn’t amputate *all* our toes? How poetic. Canagliflozin: the drug that makes your kidneys flush sugar… and your feet flush into a plastic bag.
It’s not a medication. It’s a metaphysical experiment in bodily betrayal. The FDA’s ‘benefits outweigh risks’ mantra is just the corporate version of ‘it’s not a bug, it’s a feature.’
Meanwhile, Jardiance sits there like a saint in a white coat, quietly saving limbs while Invokana whispers sweet nothings to your capillaries… until they give up and die. How very Christian of them.
Sam Clark
December 18, 2025Thank you for this comprehensive and clinically nuanced overview. As a primary care physician with over 15 years managing patients with type 2 diabetes, I can attest to the importance of individualized risk stratification.
For patients without peripheral vascular disease, neuropathy, or prior ulcers, canagliflozin remains a valuable tool-particularly in those with established cardiovascular or renal disease. However, I have implemented a mandatory pre-prescription protocol: ABI screening, podiatry referral for any sensory deficit, and a signed consent form acknowledging the amputation risk.
The data is unequivocal: the risk is real, but narrowly distributed. The key is not avoidance, but vigilance. We must not abandon a beneficial therapy due to fear, but we must also not prescribe it blindly. Precision medicine is not a buzzword-it is a responsibility.
Jessica Salgado
December 18, 2025I had a friend who lost her big toe on canagliflozin. She didn’t even feel the blister. Didn’t feel the infection. Didn’t feel the decay creeping up her foot like a slow tide. She thought it was just ‘dry skin.’
She’s now on Jardiance. Her A1c is stable. Her foot? Still whole. Her anxiety? Still there.
But here’s the thing-I’ve been on this drug for two years. No issues. My feet are fine. I check them every night. I wear socks to bed. I don’t walk barefoot. I don’t ignore redness. I don’t wait.
So… is it dangerous? Yes. For some. Is it a death sentence? No. But it’s a warning bell. And if you’re not listening? You’re already too late.
amanda s
December 19, 2025THIS IS WHY WE CAN’T HAVE NICE THINGS IN AMERICA! The FDA is a bunch of corporate lapdogs! They let this drug through because the CEO of Janssen donated to the right senators! You think they care about your toes? They care about quarterly earnings!
And now they want you to believe ‘Jardiance is safer’? HA! Same company! Same lab! Same poison, just repackaged with a prettier label!
Stop taking these drugs. Go keto. Go fasting. Go natural. Or just accept that your government sold your feet to Big Pharma. You’re not a patient. You’re a revenue stream.
Peter Ronai
December 20, 2025Oh wow. A 5.5 vs 2.8 rate? That’s not a risk-that’s a massacre waiting to happen. And you’re telling me people are still taking this? You must be one of those ‘trust the science’ zombies who don’t read the fine print.
Let me guess: you also think glyphosate is ‘safe in small doses’ and that vaping is ‘less harmful than cigarettes.’
Newsflash: if a drug causes amputations in 1 in 200 patients, it’s not a ‘trade-off.’ It’s a crime. And anyone prescribing it without a full vascular workup is negligent. Or complicit.
Jigar shah
December 21, 2025Interesting analysis. I’m from India, and here, SGLT2 inhibitors are becoming popular due to cost-effectiveness. But we rarely do ABI testing-most clinics don’t have the equipment. Many patients are unaware of foot care.
Do you think the risk profile is the same in populations with higher rates of undiagnosed PAD? We have a lot of late-stage diabetic patients presenting with foot ulcers. Would canagliflozin be riskier here than in the US population studied?
Also, is there any data on how often patients actually get foot exams after starting the drug? Or is it just ‘prescribe and forget’?
Kent Peterson
December 22, 2025So… let me get this straight: a drug that causes amputations… is still on the market… because it helps your kidneys? That’s not a benefit-that’s a moral hazard.
And you’re telling me to ‘check my feet daily’? Like I’m supposed to be a full-time podiatrist on top of my 60-hour workweek? Who’s gonna do that? The nurse? The AI chatbot?
Also-why is Jardiance ‘safer’? Did they just add a placebo pill to the bottle? Or did they just stop testing for amputations?
And don’t give me that ‘it’s not for everyone’ crap. If a drug can kill your foot, it should be banned. Period. End of story. No ‘buts.’ No ‘if you’re healthy.’ No ‘FDA says.’
My foot? It’s still here. But only because I refused the drug. And I’m not proud. I’m terrified.
Josh Potter
December 22, 2025bro i was on invokana for a year and i swear to god my toes were tinglin’ like i was walkin’ on static carpet. i didn’t say nothin’ ‘cause i thought it was ‘normal’ or somethin’. then one day i saw a lil’ red spot near my big toe-didn’t hurt, didn’t itch. just… there.
went to the doc, they freaked out. said if i waited another week, i might’ve lost it. switched me to farxiga. no more tinglin’. no more scares.
so yeah. if you feel ANYTHING weird on your feet? stop googlin’ and go see someone. your toes ain’t replaceable. and no, ‘i’m young’ don’t matter. diabetes don’t care how old you are.
Evelyn Vélez Mejía
December 23, 2025The tragedy of canagliflozin is not its mechanism-it is our collective failure to treat diabetes as a systemic disease, rather than a glycemic metric to be optimized.
We have elevated blood sugar to a moral imperative, while relegating vascular integrity, neural sensation, and tissue perfusion to the periphery of clinical concern. The amputation risk is not an anomaly-it is the inevitable consequence of a medical paradigm that reduces the body to isolated variables.
Canagliflozin is not the villain. It is the mirror. It reflects our arrogance: that we can manipulate physiology without consequence, that we can trade a toe for an A1c, that we can outsource vigilance to the patient while the system remains inert.
To prevent amputations, we must first stop treating diabetes as a number-and start treating it as a lived, embodied experience.
Jane Wei
December 23, 2025my grandma’s on this med. she’s 78, has neuropathy, and her feet look like cracked leather. she didn’t even know she could get amputated from it. i had to google it and show her the FDA warning.
she cried. then she called her doctor and got switched to jardiance.
we didn’t know. nobody told us. that’s the scariest part.
Nishant Desae
December 24, 2025hi everyone, i just wanted to say that i’ve been living with type 2 for 14 years now, and i’ve seen so many friends lose toes, feet, even legs because no one ever talked about foot care. i was on canagliflozin for a while too, and i didn’t know what to watch for. i just thought ‘if my sugar’s low, i’m good.’
but then i started checking my feet every night with a mirror, even if i couldn’t feel anything. i found a blister once, and i went to the podiatrist right away. they said if i waited, it could’ve turned into something serious.
now i’m on farxiga, and my feet are okay. i still check them. i still wear good shoes. i still don’t walk barefoot. i don’t want to be one of those stories.
if you’re reading this, please-don’t wait until it’s too late. your feet are your freedom. protect them like your life depends on it-because it does.
Jody Patrick
December 24, 2025Stop taking it if you have any foot numbness or sores. Simple. No drama. Just do it.
Radhika M
December 25, 2025Canagliflozin: bad for feet. Jardiance: good for heart and feet. Farxiga: good for heart and feet. So if you have risk factors, pick one of the others. Easy.
Also, check your feet every day. Even if you think you can’t feel anything. Use a mirror. Look. Touch. Ask someone to help.
One small sore can turn into a big problem fast. Don’t wait. Don’t ignore. Just act.
Philippa Skiadopoulou
December 26, 2025Amputation risk is dose-dependent and primarily confined to patients with pre-existing peripheral arterial disease. The data supports a risk-benefit analysis, not blanket avoidance. Screening with ABI and podiatric assessment prior to initiation is both feasible and cost-effective. The focus should remain on patient education and structured monitoring, not fear-based discontinuation.
Continued use of canagliflozin in low-risk patients remains clinically justified, provided appropriate safeguards are in place.