Cystic fibrosis isn't just another lung condition. It’s a genetic disease that changes how your body handles salt and water, turning mucus from slippery to sticky-clogging lungs, pancreas, and other organs. For decades, it meant a short life, frequent hospital stays, and hours of daily treatments. But today, that story is changing. Thanks to new drugs that fix the root cause, not just the symptoms, people with cystic fibrosis are living longer, feeling better, and doing more than ever before.
What Exactly Is Cystic Fibrosis?
Cystic fibrosis (CF) is caused by mutations in the CFTR gene, which controls how chloride moves in and out of cells. When this gene is broken, the body makes thick, sticky mucus instead of the thin, slippery kind it needs. This mucus builds up in the lungs, pancreas, liver, and reproductive system. In the lungs, it traps bacteria, leading to constant infections and scarring. In the pancreas, it blocks enzymes needed to digest food, causing malnutrition. For men, it often means infertility from birth.
It’s inherited-both parents must carry a faulty copy of the gene for a child to have CF. Carriers don’t show symptoms, so many people don’t know they’re at risk until a child is diagnosed. Newborn screening, now standard in all 50 U.S. states since 2010, catches most cases early. The most common mutation, F508del, affects about 70% of people with CF worldwide. But there are over 2,000 known mutations, and not all respond the same way to treatment.
How CF Affects the Body-Beyond the Lungs
Most people think of CF as a lung disease. But it’s a whole-body condition. About 85% of people with CF have pancreatic insufficiency, meaning they need to take digestive enzymes with every meal-often 6 to 12 capsules a day. Without them, nutrients aren’t absorbed, leading to poor growth and weight loss.
Liver problems occur in about 30% of patients, where thick bile blocks ducts and causes scarring. Sweat tests are the gold standard for diagnosis: people with CF have sweat chloride levels above 60 mmol/L, far higher than normal. And while CF doesn’t directly cause diabetes, nearly half of adults with CF develop it due to pancreatic damage.
Respiratory failure is still the top cause of death, responsible for 85% of CF-related deaths. Chronic infections with bacteria like Pseudomonas aeruginosa and Staphylococcus aureus wear down the lungs over time. Even with antibiotics and daily airway clearance, many people face progressive lung damage.
The Old Way: Daily Grind, No Cure
Before 2012, treatment was all about managing symptoms. A typical day for an adult with CF could include:
- 90 minutes of chest physiotherapy or breathing devices to loosen mucus
- 4 to 6 different inhaled medications-bronchodilators, antibiotics, mucolytics
- Enzyme capsules with every meal and snack
- Vitamins, high-calorie shakes, and strict diets to fight malnutrition
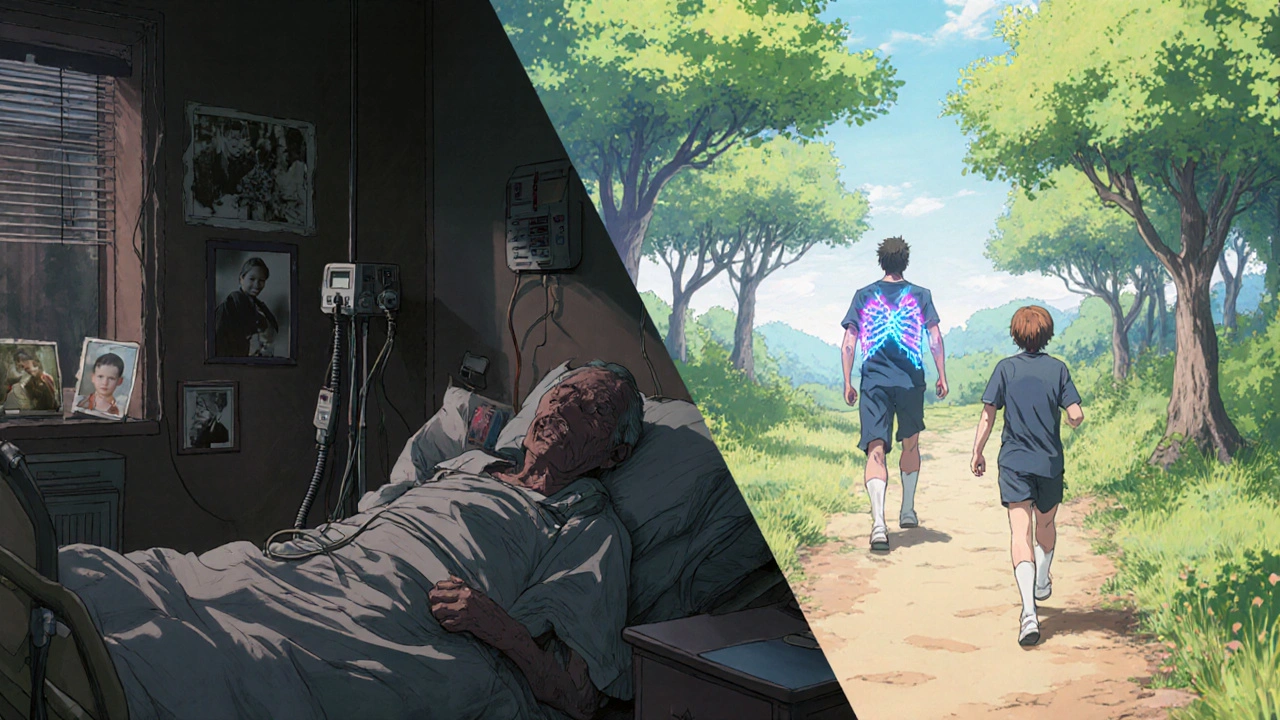
That’s 2 to 3 hours a day, every single day. Adherence was tough. Studies show only 65-75% of patients stuck with the full routine. Miss a day, and infections could flare up. Hospital visits were common. In 1960, the median life expectancy was just 14 years.
The Game Changer: CFTR Modulators
The first real breakthrough came in 2012 with ivacaftor (Kalydeco). It was designed for people with the rare G551D mutation-and it worked. In trials, patients improved lung function by over 10%. But it helped only about 4% of the CF population.
Then came the triple combo: elexacaftor/tezacaftor/ivacaftor (Trikafta), approved in 2019. This drug fixes the most common mutation, F508del, which affects 70% of people. In clinical trials, Trikafta boosted lung function by 13.8% and cut pulmonary exacerbations by 63%. Suddenly, people were coughing less, gaining weight, needing fewer antibiotics, and spending less time on airway clearance.
One 28-year-old patient shared on a CF forum that after starting Trikafta, their daily airway clearance dropped from 90 minutes to 20. That’s not just a convenience-it’s a life reset.
By 2023, 90% of people with CF in the U.S. had access to at least one modulator. The median predicted survival jumped to 50.9 years-up from 14 in 1960. For the first time, more adults than children live with CF. In 1990, only 27% of patients were over 18. Now, it’s 52%.
Who Doesn’t Benefit? The 10% Left Behind
But not everyone can use these drugs. About 10% of people with CF have mutations that current modulators can’t fix-mostly Class I mutations, where the CFTR protein doesn’t get made at all. For them, the old daily grind remains. Some still rely on lung transplants, which come with their own risks and lifelong immunosuppression.
Researchers are working on solutions. mRNA therapies like Ataluren aim to trick cells into making functional CFTR protein even with nonsense mutations. CRISPR gene editing is being tested in early trials to correct the faulty gene directly. New inhaled antibiotics are being developed to tackle stubborn Pseudomonas infections. The Cystic Fibrosis Foundation has pledged $100 million to target this gap with its ‘Path to a Cure’ initiative.
The Cost Problem: A Treatment Too Expensive for Many
These drugs are miracles-but they’re also among the most expensive in the world. Trikafta costs around $300,000 per year in the U.S. Even with insurance, many patients pay $1,200 a month out of pocket. A 2022 survey found 42% of modulator users reported financial strain.
Global access is worse. In high-income countries, 85% of eligible patients get modulators. In the EU, it’s 45%. In low- and middle-income countries, it’s less than 10%. The World Health Organization calls this inequity a crisis. Over 75% of CF deaths now happen in places where these drugs aren’t available.
Vertex Pharmaceuticals, which makes all approved modulators, holds 95% of the market. The Cystic Fibrosis Foundation helped fund the early research with $150 million in venture philanthropy back in 2000. That investment paid off-but the pricing model hasn’t kept pace with global need.
Side Effects and Real-Life Trade-Offs
Modulators aren’t perfect. Some patients report liver enzyme spikes, requiring temporary shutdowns. A 2023 study found 3.2% of users had to stop treatment due to liver issues. Others report headaches, rashes, or changes in vision. For some, the benefits outweigh the risks. For others, the side effects are too much.
One Reddit user described how their child developed severe nausea and weight loss on Trikafta. After switching back to older therapies, they regained stability-even if it meant returning to 2 hours of daily treatments. Personal experience matters more than trial data.
What’s Next? The Future of CF Care
The FDA approved Trikafta for kids as young as 2 in early 2023. That means more children are growing up with better lung health from the start. Early intervention could prevent much of the damage that used to accumulate over years.
Research is shifting from treating symptoms to preventing them entirely. Gene editing, mRNA therapies, and new anti-infectives are in active trials. The goal isn’t just longer life-it’s better life, with fewer hospital visits, less medication, and more freedom.
Meanwhile, care centers across the U.S. (260 accredited sites) and global networks like CF Buddy Connect offer support, education, and peer advice. The International Cystic Fibrosis Conference brings together doctors, researchers, and patients to share what’s working-and what’s not.
Living With CF Today
CF is no longer a death sentence. It’s a chronic condition-with a treatment revolution behind it. For many, it’s now manageable. For others, the fight continues. The tools exist to transform lives. But access, cost, and equity remain the biggest hurdles.
If you or someone you know has CF, the message is clear: Talk to your care team about modulator eligibility. Even if you’ve been on old therapies for years, you might qualify now. And if you don’t, you’re not alone-research is racing to catch up.
Can cystic fibrosis be cured?
There is no cure yet, but CFTR modulator therapies can fix the underlying defect for most people. These drugs don’t eliminate the gene mutation, but they restore enough function to dramatically improve health and life expectancy. Research into gene editing and mRNA therapies may one day offer a true cure, but those are still in early testing.
How do CFTR modulators work?
CFTR modulators are precision drugs that help the faulty CFTR protein work better. Some, like ivacaftor, act as a “gate opener” to help chloride move through the channel. Others, like elexacaftor and tezacaftor, act as “chaperones” to help the protein fold correctly and reach the cell surface. Together, they restore salt and water balance, thinning mucus and reducing infections.
Is cystic fibrosis only a childhood disease?
No. Thanks to new therapies, more than half of all people with CF in the U.S. are now adults. In 1990, only 27% were over 18. Today, that number is 52%. With better lung function and fewer hospitalizations, many people with CF are living into their 50s, 60s, and beyond.
Why are CFTR modulators so expensive?
They’re rare disease drugs developed over decades with high research costs. Vertex Pharmaceuticals, the sole manufacturer, holds exclusive rights under orphan drug laws. The Cystic Fibrosis Foundation helped fund early research, but pricing decisions were made by the company. While the drugs save lives, their cost creates major access gaps, especially outside wealthy countries.
What if I have a rare CF mutation?
About 10% of people have mutations not covered by current modulators. Your care team can test your specific mutation and enroll you in clinical trials for emerging therapies like mRNA treatments or gene editing. Organizations like the Cystic Fibrosis Foundation actively recruit for these trials and offer support to help you participate.
Can I still get a lung transplant if I’m on modulators?
Yes, but it’s less common now. Transplants are still an option for people whose lungs fail despite modulator therapy. However, because modulators slow lung decline, fewer patients need transplants. Transplant candidates are carefully evaluated, and modulator use doesn’t disqualify you-it may actually improve your chances by keeping you healthier before surgery.




9 Comments
Shante Ajadeen
November 12, 2025Just read this and had to take a second. My cousin has CF and started Trikafta last year. She went from barely making it through work to hiking on weekends. No more 90-minute breathing sessions. It’s wild how much one drug can change everything.
Still, it breaks my heart thinking about people who can’t even get access to it. We’re talking life or death here, and it’s all about money.
They need to fix this. Not just in the US - everywhere.
dace yates
November 12, 2025I’ve been reading up on CFTR modulators since my nephew got diagnosed. I didn’t realize how many mutations exist. The fact that 10% are left out is terrifying. I hope mRNA therapies work - it feels like the next real leap.
Also, why does it take so long to test new drugs for rare mutations? It shouldn’t be this slow.
Danae Miley
November 14, 2025Let’s be clear: Vertex isn’t a charity. They made billions off taxpayer-funded research and orphan drug exclusivity. The $300,000 price tag isn’t ‘R&D cost’ - it’s greed wrapped in a lab coat.
WHO says 75% of CF deaths happen where these drugs aren’t available? That’s not a tragedy - it’s a crime. And the fact that we’re still debating ‘affordability’ instead of forcing price caps is disgusting.
Charles Lewis
November 15, 2025It is both remarkable and sobering to observe the paradigm shift that has occurred in the clinical management of cystic fibrosis over the past two decades. From a condition that was, in the mid-20th century, universally fatal before adolescence, we now find ourselves in an era where the median life expectancy exceeds 50 years.
This transformation is attributable not merely to incremental improvements in symptomatic care, but to a fundamental reengineering of therapeutic strategy - from palliation to correction. The advent of CFTR modulators represents a landmark in precision medicine, demonstrating that targeting the root molecular pathology can yield transformative outcomes.
However, the ethical imperative to ensure equitable access cannot be overstated. The disparity in global availability, particularly between high-income nations and low- and middle-income countries, constitutes a profound injustice. The international community must prioritize mechanisms for technology transfer, tiered pricing, and generic development to prevent this medical advancement from becoming a privilege of wealth rather than a right of health.
Renee Ruth
November 16, 2025Okay but have you seen the Reddit thread where a mom said her kid lost 15 pounds on Trikafta and started hallucinating? And then the doctors were like ‘it’s normal’? Yeah. This isn’t a miracle. It’s a gamble with side effects no one talks about.
And now everyone’s acting like it’s the end-all-be-all. What about the people who can’t tolerate it? Are they just supposed to ‘try harder’?
Also, Vertex stock is up 400% since 2019. Coincidence? I think not.
Samantha Wade
November 16, 2025Let’s not romanticize this. Yes, Trikafta is revolutionary - but it’s not a cure. And the 10% who don’t benefit? They’re not ‘less deserving.’ They’re the reason we can’t stop pushing for gene editing and mRNA solutions.
The Cystic Fibrosis Foundation’s $100 million ‘Path to a Cure’ initiative is a start - but we need global funding, not just American charity. Insurance companies in the U.S. still deny coverage based on ‘off-label’ use. That’s unacceptable.
If you have CF and are on older therapies - don’t give up. Your care team might have new options you haven’t considered. And if you’re reading this and you’re healthy - advocate. Call your reps. Demand price transparency. This isn’t just medical - it’s moral.
Elizabeth Buján
November 17, 2025im just so happy that people with cf are living longer 😭
like… i had a friend in high school with it and she was always tired, always in the nurse’s office. now she’s traveling, got a dog, posted a pic of her baking bread last week.
but why is it so expensive?? i dont get it. its not like they invented a new kind of pizza. its fixing a broken part of the body. why does that cost more than a car?
we need to fix this. for everyone.
Andrew Forthmuller
November 17, 202510% left out. That’s 20k people. Just in the US. And we’re talking about drugs that could save them. Why isn’t this bigger news?
vanessa k
November 19, 2025My sister’s been on the old routine for 15 years. She’s 32. She does her 2-hour routine every day. No sugar-coating - it’s exhausting. But she’s still here.
I just want people to know - she’s not ‘behind.’ She’s not ‘failing.’ She’s surviving. And if Trikafta doesn’t work for her? That doesn’t make her less worthy of care.
Everyone deserves a shot. Even the ones who don’t fit the mold.